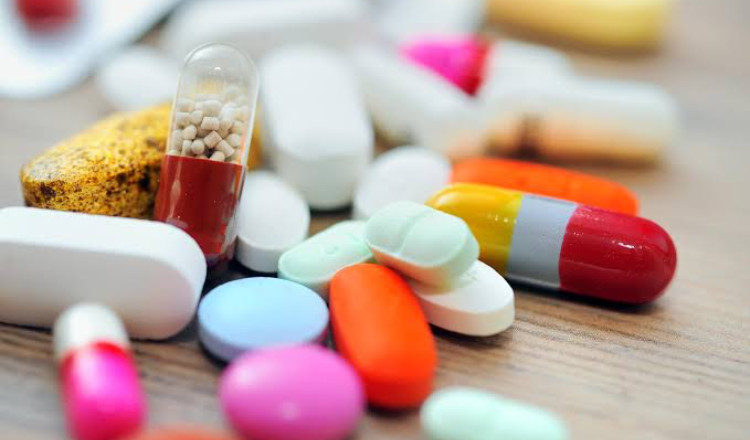
The Indian government has banned the production and export of drug combinations containing tapentadol and carisoprodol. This decision follows reports that unapproved versions of these drugs were being sent to West African countries, leading to a rise in opioid addiction. A BBC investigation revealed that Mumbai-based Aveo Pharmaceuticals was exporting these unapproved combinations to nations like Nigeria and Ghana, where they were misused.
Tapentadol is a strong painkiller, while carisoprodol is a muscle relaxant. Although both are approved individually in India, their combination is not authorized. The Drugs Controller General of India (DCGI) has instructed all state drug authorities and customs offices to cancel any permissions for manufacturing or exporting this combination.
In response to these findings, a joint team from the Central Drugs Standard Control Organization (CDSCO) and Maharashtra's regulatory authority audited Aveo Pharmaceuticals. This led to a halt in all operations at the company's facilities. Authorities seized over 13 million tablets and capsules, along with 26 batches of active ingredients, to prevent further distribution.
The Indian government emphasizes its commitment to maintaining high standards in drug safety and regulation. While supporting the global supply of legitimate medicines, it is taking strong actions against the illegal export of unapproved and potentially harmful drugs.

Tamil Nadu to Appoint 2,642 Doctors Starting February 12
Doctors in Hyderabad Remove Pen Cap from Man's Lungs After 21 Years
Doctors Criticize NEET PG 2025 Two-Shift Exam Plan, Warn of Repeat Issues
Two Haryana Doctors Die in Car Accident Involving Nilgai
With deep sadness & heavy heart informing the sad demise of IMA Cherthala Branch member Dr Seena P (49), Assistant professor Of Dermatology at Alappuzha Medical college & Wife of Dr. Vivek Kumar Ravi (Deputy Director DHS ) Heartfelt condolences IMA ALAPPUZHA BRANCH
We have various options to advertise with us including Events, Advertorials, Banners, Mailers, etc.